|
阅读:2923 ,发布于2023/11/15 10:44
Understand what drives peptide synthesis purity, speed, and waste
generation
Introduction
Anyone that has performed peptide synthesis, either manual or automated,
realizes quickly that each peptide is different. Peptide sequences can vary in
length, contain many different amino acids or a few of the same, be soluble or
insoluble, contain disulfide bonds, and many more. With any standard synthesis
protocol, the “easy” peptide results in both high crude purity and high
purified product yield. While a difficult peptide using a standard synthesis
protocol delivers crude peptides with low purity that require more downstream
work and lower yields of the purified product (or in some situations, no mass
identification of the target peptide).
When performing peptide synthesis, it is not uncommon for a peptide
containing only 10 amino acids to be considered more “difficult” to synthesize
than a much longer 25+ amino acid peptide. Overall success with synthesizing a
specific peptide is generally impacted by:
-
Peptide Length: the total number
of amino acids in the peptide sequence.
-
Peptide Sequence: the specific
combination and order of the amino acids in the sequence.
-
Synthesis Protocol: The set of
steps that together add amino acids to the peptide sequence. These include
deprotection washes, coupling time, as well as the number of cleaning
washes after both deprotection and coupling.
Of these factors, the Synthesis Protocol is the only way one can control
the conditions of their syntheses and optimize their protocol to deliver pure
peptides in a time-efficient manner.
Peptide Synthesis Protocols
When making a peptide,
users must balance the purity and yield while also optimizing for synthesis
speed and reducing waste for their workflow. Heating the synthesis can allow
users to lower the times for deprotection and coupling, leading to faster cycle
times. This can be done in a variety of ways including conduction heating and
microwave heating. For the best results, heating needs to be delivered
uniformly and to consistent temperatures. Conduction heating is scalable from
20 mL up to commercial-size reaction vessels 500L+. It avoids racemization
caused by spikes and hotspots typically associated with microwave heating.
Conduction heating is also a much more cost-efficient heating method when
compared to expensive microwave heating technology.
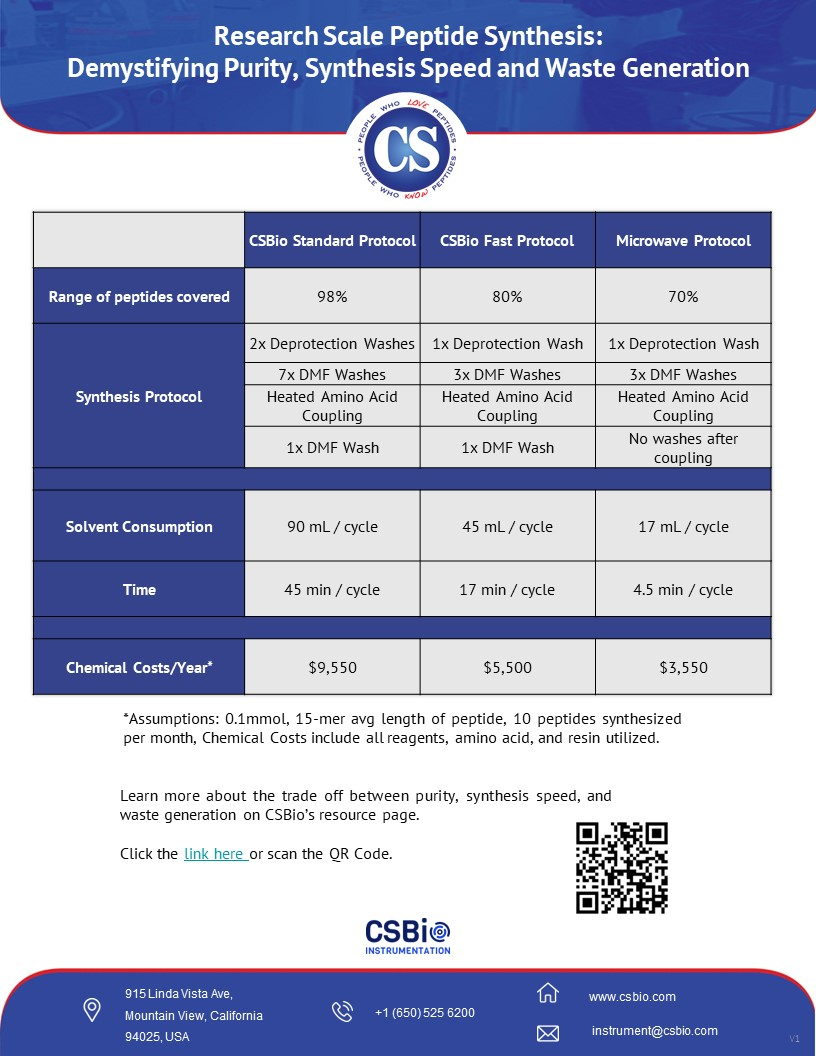
Synthesis speed and
waste also go hand in hand as shown in the chart. It is easy to see that if a
protocol contains more DMF washes, 8 vs 3, the protocol with 8 washes will be
expected to take longer and generate more waste.
That bodes the question:
Why do two instruments aiming to complete the same objective differ so much in
their standard protocol?
Higher Purity - The
protocol with 8 washes is more likely to deliver a higher purity peptide due to
maintaining a cleaner synthesis. The washes after deprotection are essential
for ensuring that all the Fmoc group is completely gone before coupling. Doing
washes after coupling ensure that your main peak will be your main peak,
leaving no room for cross-contamination between amino acid cycles. Look at
our case study of
the synthesis of a 132-mer peptide on CSBio’s research scale peptide
synthesizers
Universally
Applicable - A user also benefits from an automated peptide synthesizer
that is preloaded with protocols that will work well on nearly all peptides,
regardless of sequence or length. The protocol with 8 washes can be run on a
wide range of peptide sequences and expect a successful synthesis, not for a
fraction of the peptide sequences. Users of the CSBio Peptide synthesizers can
easily choose between a conservative or aggressive washing protocol giving them
the ability to reduce the number of washings after deprotection and coupling to
improve synthesis speed and reduce waste.
Peptide
Synthesis - Synthesis Protocols and the impact on Purity, Speed, and Waste
Generation
Cost to Perform Peptide Synthesis
What’s the raw material cost to run a
peptide synthesizer? Well, most research labs will synthesize ~10 peptides a
month at the 0.1 mmol scale, usually averaging about 15 amino acids in length.
Then it’s largely dependent on the synthesis protocol utilized and the type of
raw materials used. Some peptide synthesizer manufacturers require special
amino acids and resins to run their synthesis protocol, such as His(Boc) that
can be 7 times more expensive than standard His(Trt), or PEG-PS resin that can
be twice as expensive as Rink Amide MBHA resin.
When considering the synthesis protocols,
the amino acids, resin, and all the reagents and solvents, a typical cost per
year will range between $5k to $10k. This considers purchasing the amino acids
in 100g bulk (not pre-packaged cartridges), and does not include any disposable
or consumable items that the synthesizer manufacturer may require. If you're
interested in learning about the details of the cost to perform peptide
synthesis and these calculations, just reach out to us.
As users can see, while there may be a
marginal potential cost savings from the reduced waste of the microwave
protocol, this doesn’t factor in re-running the synthesis in situations that
the target peptide is not found. Even without the re-synthesis situation,
looking at the total cost per run is negligible when factoring in the much
higher purchase price of an expensive microwave synthesizer.
With a CSBio Peptide Synthesizer, users are
confident that the provided protocols deliver high-quality results on difficult
peptides while allowing users to easily change the protocol to manage synthesis
speed and waste. Reach out to us at instrument@csbio.com,
schedule a call to
learn more, or just ring us directly at +1 650 525 6200 if you’re
interested in discussing your peptide synthesizer needs.
About CSBio: For over 30 years, CSBio, a leading peptide and peptide
synthesizer manufacturing company located in Silicon Valley, California, has
been providing cGMP peptides and automated peptide synthesizers to the global
pharmaceutical community. CSBio’s peptide products and peptide synthesizers can
be found in production laboratories, universities, and pharmaceutical companies
worldwide.
|
 沪公网安备 31011502005552号
沪ICP备13005633号-1
沪公网安备 31011502005552号
沪ICP备13005633号-1