|
阅读:903 ,发布于2023/11/15 10:47
In this case study, we talk about CSBio's peptide synthesizer platform
and knowledge that allows companies to utilize our peptide synthesizers during
the early days of drug discovery, all way through commerical scale
manufacturing
Introduction
When a multinational pharmaceutical company initiated their in-house
manufacturing of peptides in the early phases of drug discovery, they came to
CSBio for their instrumentation needs. As a manufacturer of custom peptide
synthesizers from small scale to large scale manufacturing, this pharmaceutical
company knew they had a partner that could meet all of their instrumentation
needs from drug discovery to commercial manufacturing for process development
and scale up ease.
Brief
During drug discovery
for lead optimization, this pharmaceutical company determined they needed a
reliable small scale synthesizer to produce peptide analogs which would be used
in testing to determine the best analog for scale-up and clinical trials. As a
fair amount of material would be required for this testing (20-100 mg) and
flexibility in the synthesis protocol was important, they turned to the CS136
which enabled their discovery lab to rapidly synthesize dozens of peptide
analogs in a short amount of time with enough material produced for multiple
tests and assays.

CSBio
CS136M
Once a primary peptide drug candidate was selected, the peptide chemists
at this pharmaceutical company were tasked with making a continuous supply of
high purity peptide for toxicological studies, PK studies, formulation work and
additional assay testing. Grams of peptide were now required, so the
pharmaceutical company set up a CS536 system in the process development
laboratory to accomplish this task.
To manage the scale up transition from small scale to large scale
manufacturing, a second CS536 system was purchased shortly thereafter and
customized with both a 180° inversion mixing system and overhead stirring. This
unique design was a collaboration between CSBio design engineers and the
pharmaceutical company to add an extra layer of confidence in their methodology
as they prepared for the scale up transition from inversion mixing to overhead
mixing, which would ultimately be required for large scale manufacturing of the
peptide drug.
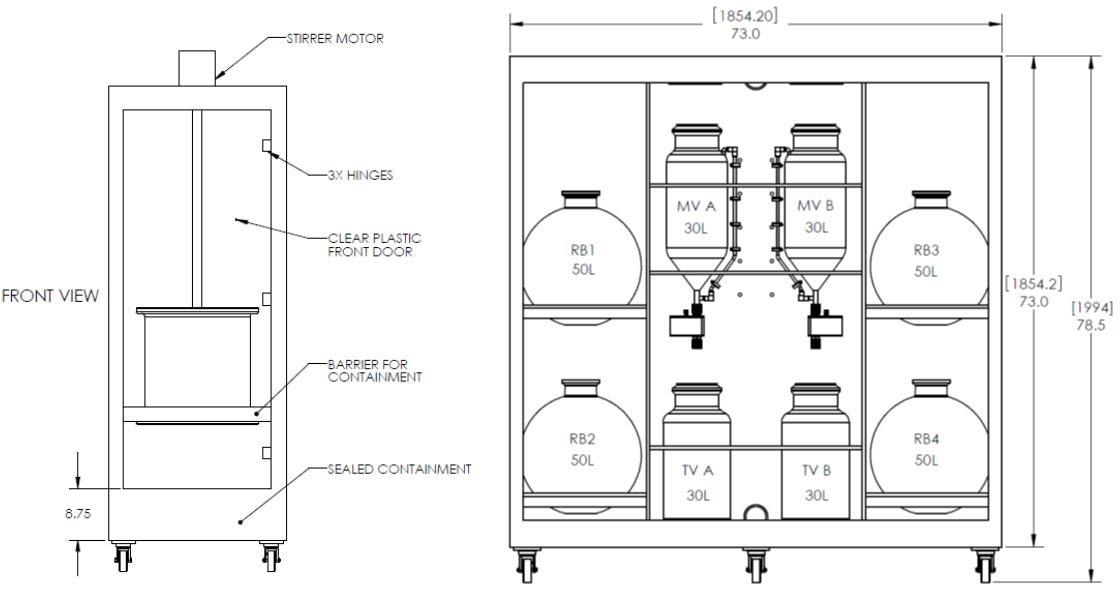
Custom
Designed CSBio CS936
As the pharmaceutical
company moved into late stage clinical trials the new task at hand was scaling
up for commercial manufacturing. CSBio and the pharmaceutical company worked
closely together to design a CS936 production scale synthesizer that would suit
their exact large scale production requirements. It was imperative that the
transition from R&D scale to process scale to manufacturing was as seamless
as possible. As all CSBio synthesis systems use the same 21 CFR Part 11
compliant CSPEP software®, the transition from milligrams to grams to kilograms
was made much easier as the synthesis protocols could be transferred up the
production scale.
The CS936 unit was synthesizing
manufacturing runs immediately upon installation/operational qualification as
there was virtually no learning curve for the manufacturing group. CSBio has
purposely designed our systems to make scale up from R&D to commercial
manufacturing as easy as possible.
Conclusion
CSBio provides customers with peptide synthesizers that fit their exact
needs throughout the lifecycle, whether it’s during early drug discovery or
late stage commercial manufacturing. By working intimately with customers
coupled with over 30 years of peptide and instrumentation knowledge, customers
can trust they have a partner to ensure a successful project.
Interested in any of our peptide synthesizers? Get in touch with us.
|
 沪公网安备 31011502005552号
沪ICP备13005633号-1
沪公网安备 31011502005552号
沪ICP备13005633号-1