|
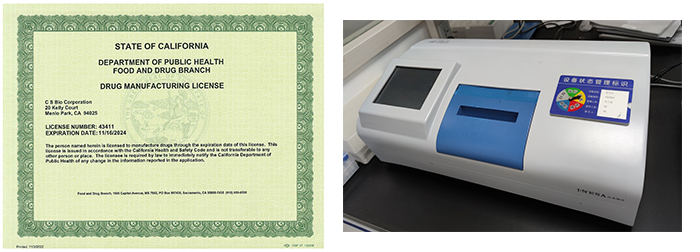
Good quality system is the CSBio corporate culture and mission. We are committed to ensure full customer satisfaction by providing quality products and services. Our staffs are highly trained, and well versed in quality standards. We guarantee that our products and services are scrutinized with the most advanced testing techniques and validation prior to shipment to our customer so that our customer is trouble-less upon receipt of products and services. We can provide Certificate of Analysis, Method of Analysis and actual quality data, shelf-life data.
Our COA will include the following data (dependent on the characteristic and properties of the product)
 Purity (HPLC) Purity (HPLC)
 Purity (UPLC) Purity (UPLC)
 Mass Mass
 NMR NMR
 Chiral Chiral
 Ion Chromatography Ion Chromatography
 Specific Rotation Specific Rotation
 Karl Fisher Titration Karl Fisher Titration
 Acid-Base titration Acid-Base titration
 UV/Vis UV/Vis
 Metallic Analysis Metallic Analysis
 Elemental Analysis Elemental Analysis
 Amino Acid Analysis Amino Acid Analysis
Besides above mentioned quality systems, we also try our best to support our customers in meeting the GMP or new drug registration requirement by providing the following technical documents:
 Vendor Questionaire Vendor Questionaire
 TSE/BSE Declaration TSE/BSE Declaration
 Non-GMO Declaration Non-GMO Declaration
 Route of Synthesis Route of Synthesis
 Melamine Declaration Melamine Declaration
 Change Declaration Change Declaration
 Potential Impurity List Potential Impurity List
 Solvents Declaration Solvents Declaration
 Instrumentation list Instrumentation list
 Raw Material List Raw Material List
 SDS SDS
For enquiries or comments on quality on productsand services, please contact: quality@csbiochina.com



|
 沪公网安备 31011502005552号
沪ICP备13005633号-1
沪公网安备 31011502005552号
沪ICP备13005633号-1