|
Hits:218 , posted at 2026/1/29 16:05
The Origin of GLP-1 Drug Peptides
In the 1960s, scientists discovered human GLP-1 receptor agonists. GLP-1 is an intestinal
peptide hormone secreted by L-cells of the small intestinal mucosa. It acts on receptors in specific areas of the
brain to efectively inhibit the appetite center, enhance satiety, andreduce food intake5 . GLP-1 regulates glucose metabolism through multiple mechanisms to achieve blood sugar control and weight loss.
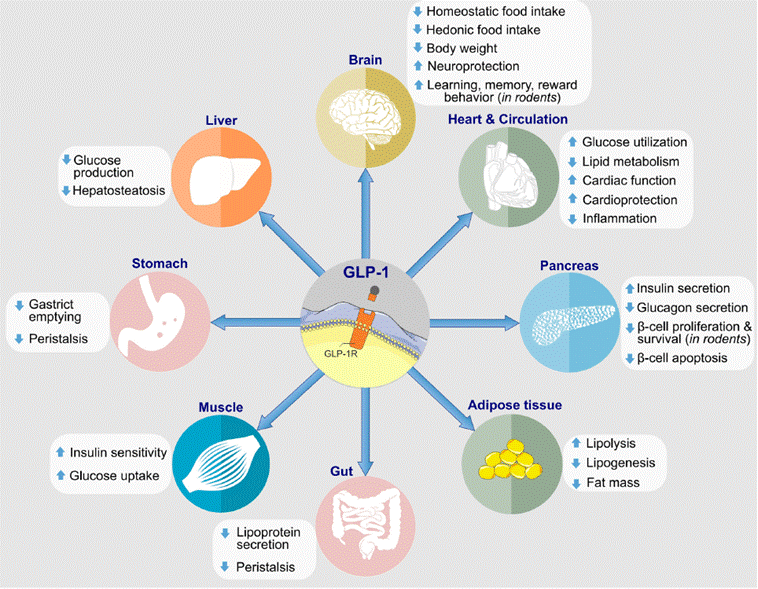
Figure 1: Biological efects of
GLP-1 on target tissues
(Source: Frontiers in Endocrinology)
Initially known primarily as a blood sugar regulator, GLP-1 is now recognized for its roles in treating
diabetes, cardiovascular disease, Alzheimer’s, depression, kidney and liver diseases, and arthritis.
Liraglutide
Because the plasma
half-life of natural human
GLP-1 is extremely short (less than 2 minutes), scientists began developing long-acting GLP-1 analogues. In the early 21st century, the Danish company Novo Nordisk
successfully developed Liraglutide, a peptide drug with 97% homology to human GLP-1. It was approved in the EU and US in 2009 and 2010 respectively, and launched in China in 2011.
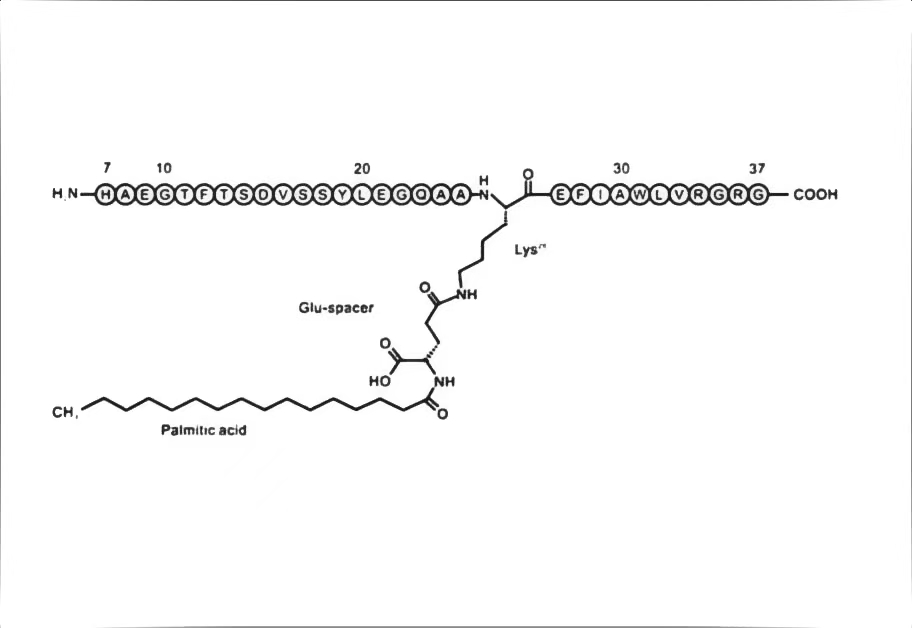
Figure 2: Structural formula
of Liraglutide
The N-terminal sequence of natural GLP-1 (His-Ala-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu...)
(7-36 amide) contains a key cleavage site: Ala-Glu. This site is specifically recognized by the DPP-4 enzyme, which hydrolyzes and removes the first 8 amino acids. This process transforms active GLP-1 into an inactive fragment, resulting in its short half-life. Furthermore, the wide
distribution of DPP-4 in vascular
endothelial cells and lymphocytes
accelerates its clearance, making
natural GLP-1 dificult to use clinically.
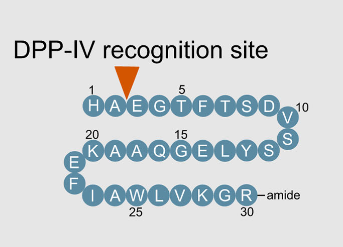
Figure 3: DPP-4 cleavage site on
GLP-1
(Source: Frontiers in Endocrinology)
Liraglutide’s design achieved two breakthroughs:
1. Palmitic Acid Side Chain: A 16-carbon fatty acid allows it to
bind reversibly to serum albumin, protecting
it from DPP-4 degradation.
2. Amino Acid Modification: Replacing Lysine at position 34 with Arginine enhanced structural stability.
These modifications extended the half-life to approximately 13 hours, allowing for once- daily subcutaneous injection.
Therapeutic Efects:
• Pancreas: Promotes
glucose-dependent insulin secretion and protects β-cells.
• CNS: Activates satiety signals in the hypothalamus and reduces cravings for high- calorie foods.
• Gastrointestinal: Delays gastric emptying.
• Cardiovascular: Improves endothelial function and inhibits atherosclerosis.
• Metabolism: Promotes "browning" of white fat and improves liver insulin sensitivity.
Semaglutide
Building on Liraglutide’s success, Novo Nordisk
developed Semaglutide, a next-generation GLP-1 analogue with 94% sequence homology. It targets type 2 diabetes and obesity with a significantly longer half-life of approximately 7 days, allowing for once-weekly injections.
Unlike many drugs, its metabolism is widespread across tissues rather than being confined to specific organs, meaning its use is not limited
by liver or kidney function.
|
Drug Name
|
Long-acting Strategy
|
Half-life
|
|
Exenatide
|
Sustained-release microspheres; sequence
modification
|
2.5
hours
|
|
Beinaglutide
|
PEGylation (Polyethylene glycol chain)
|
4 days
|
|
Dulaglutide
|
Fc fusion protein (Immunoglobulin)
|
4.7 days
|
|
Semaglutide
|
Fatty diacid side chain; amino acid
substitution
|
7 days
|
|
Glutazumab (Phase III)
|
GLP-1 peptide fused with GLP-1R antibody
|
1 month
|
Table 1: Long-acting Strategies
for GLP-1RA Drugs
(Source: Chinese Prescription Drugs)
Semaglutide is composed of a total of 31 amino acids consisting of 17 distinct types. These 17 amino acids are: histidine, alpha-aminoisobutyric acid, glutamic
acid, glycine, threonine, phenylalanine, serine, aspartic acid, valine, tyrosine,
leucine, glutamine,
alanine, lysine, isoleucine, tryptophan, and
arginine.
Structural Modification and Synthesis

• Modification Structure: The lysine residue of semaglutide contains a C18 fatty diacid modification.
• Spacer Modification: Between the lysine and this modification structure, there is a spacer composed of glutamic acid and a 2-[2-(2-aminoethoxy)ethoxy]acetic
acid dimer4 .
• Synthesis
Method: During the synthesis of semaglutide, the peptide backbone and the side chain containing the C18 fatty diacid are generally synthesized separately and then
conjugated.
• Fragment Assembly: The entire molecule is assembled from multiple fragments combined together.
Semaglutide features three critical modifications:
1. Aib Substitution: Alanine at position 8 is replaced by α-aminobutyric acid to "hide" the cleavage site from DPP-4.
2. Arg Substitution: Lysine at position 34 is replaced by Arginine.
3. C18 Side Chain: Lysine at position 26 is acylated with an 18-carbon fatty diacid and a
2xOEG spacer, allowing for tighter binding to plasma albumin and reduced
renal
filtration.
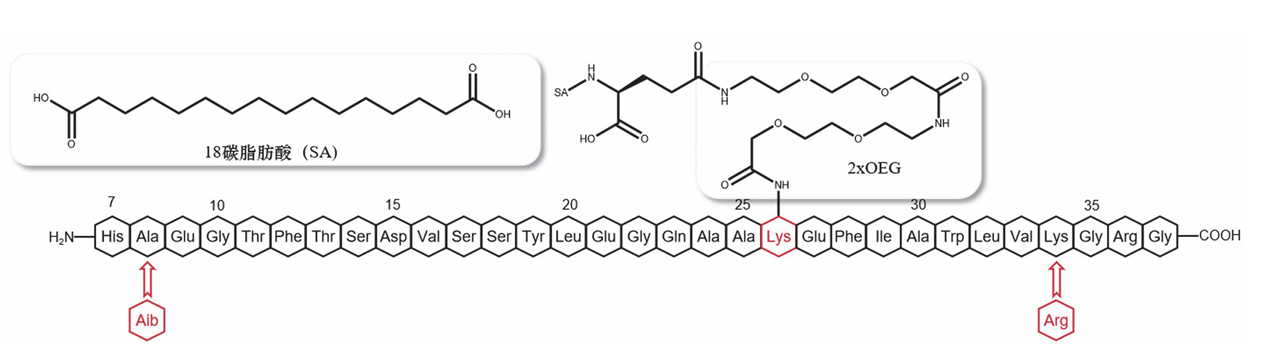
Figure 4: Simplified structure of
Semaglutide
After modification, the guanidino group on the side chain of Arginine
(Arg) at position 34, the carbonyl group on the alpha-carbon,
and the carboxyl group on the side chain of
Glutamic
acid (Glu) at position 27 achieve the specific acylation of Lysine (Lys) at
position 26 through non-covalent interactions with water molecules. This provides a solid foundation for subsequent chemical modifications.
To evade recognition by the metabolic
system, the molecule must adhere tightly to plasma albumin, which serves as an essential "transport vessel" within the body. Through the
addition of a fatty
acid side chain, an acidic
gamma-Glu group, and lipophilic 2xOEG groups, the molecule can
bind more securely to plasma albumin, thereby reducing its glomerular filtration rate4 .
Furthermore, the amino acid sequence was engineered to "hide" the DPP-4 cleavage site. By replacing Alanine at position 8 with alpha-aminoisobutyric
acid (Aib), the DPP-4
enzyme is unable to recognize the site, which significantly
enhances Semaglutide's
resistance to degradation. Additionally, the formation of numerous hydrogen bonds and hydrophobic interactions increases both the structural stability of the molecule and its binding afinity to the target
receptor.
Through these ingenious modifications, the drug's
half-life can be extended to approximately 7 days.
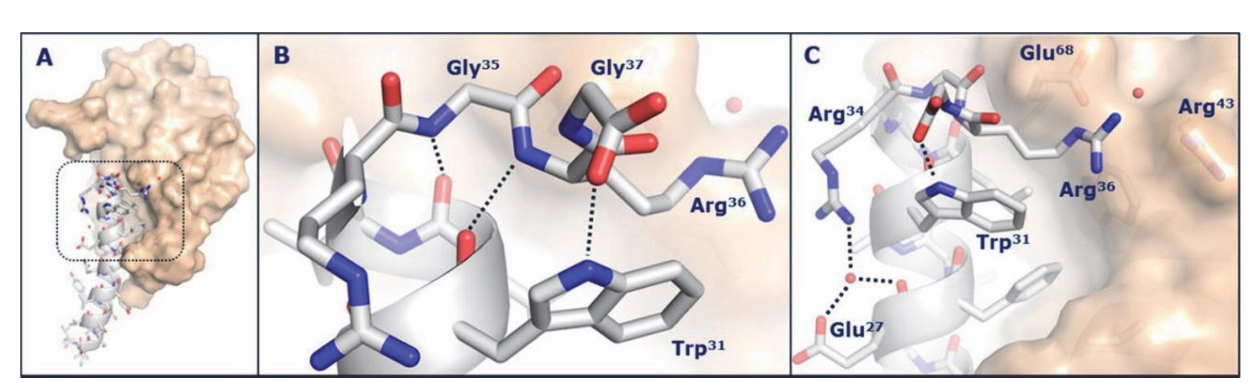
Figure
5: (A) Crystal structure of the semaglutide
peptide backbone (gray) complexed
with the GLP-1 receptor
extracellular domain (gold surface); (B, C) Magnified overall
structure.
(Source: Frontiers in Endocrinology)
Tirzepatide
Following Liraglutide and Semaglutide, the field of GLP-1 receptor agonists (GLP-1RAs) has seen a
new breakthrough: Tirzepatide. Developed by the American company Eli Lilly,
Tirzepatide is a dual agonist for both the glucose-dependent insulinotropic polypeptide (GIP) receptor and the GLP-1 receptor. The drug received approval from both the US FDA and the
European Union in 2022 for the treatment
of type 2 diabetes, demonstrating
significant glucose-lowering and weight-loss efects in multiple clinical trials.
Tirzepatide is an engineered 39-amino acid peptide with a structure based on the GIP
sequence, achieving long-acting properties through a C20 fatty acid side chain. Unlike
pure GLP-1 receptor agonists, Tirzepatide activates two types of receptors simultaneously to create a synergistic efect:
• GIP Receptor Activation: Enhances insulin secretion, improves insulin sensitivity, and may promote lipid metabolism.
• GLP-1 Receptor Activation: Inhibits appetite, delays gastric emptying, and promotes insulin secretion.
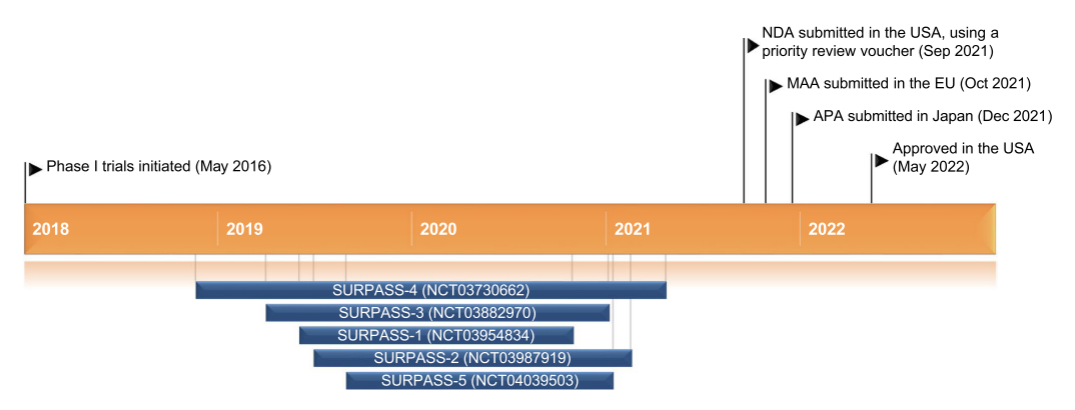
Figure 6: Key milestones in Tirzepatide treatment for type 2 diabetes
(Source: Springer Nature)
This dual-agonist mechanism allows it to outperform single GLP-1
receptor agonists in both blood sugar control and weight reduction. Tirzepatide has a half-life
of approximately 5
days, supporting once-weekly subcutaneous
injections.
In several Phase III clinical trials (such as the SURPASS series), Tirzepatide was significantly superior to Semaglutide and insulin in reducing glycated
hemoglobin (HbA1c) and body
weight. Some patients experienced a weight loss of over 20%, making it one of the most potent weight-loss medications
currently available.
Tirzepatide is currently available in an injectable format. Its common adverse reactions are similar to other GLP-1 drugs, primarily consisting of gastrointestinal issues (nausea,
diarrhea, vomiting, etc.), which are mostly mild to moderate and
typically decrease over time. Due to its
dual-receptor mechanism, more real-world
data is still needed to support its long-term safety.
Tirzepatide represents a new generation
of dual-target GLP-1/GIP drugs, ofering a new
treatment option for patients with type 2 diabetes
and obesity. In the future, with the
development of oral formulations and the expansion of approved indications, Tirzepatide is expected to play an even more significant role in metabolic disease treatment.
The Transformation from
Injection to Oral Administration
Since Semaglutide gained widespread
recognition, the pharmaceutical industry has
continued to refine GLP-1 class peptides, eventually introducing more
patient-friendly oral formulations. As peptides, these molecules have large molecular weights, are hydrophilic, and possess low permeability,
making them dificult to absorb through the gastrointestinal mucosa. Furthermore, they are easily degraded and lose pharmacological activity due to
the high levels of stomach acid and pepsin.
The ability of this drug to withstand gastric
degradation and cross the small intestinal
epithelial cells depends on a crucial partner: salcaprozate sodium (SNAC). The oral tablet is
formulated with 14 mg of Semaglutide and 300 mg of SNAC.
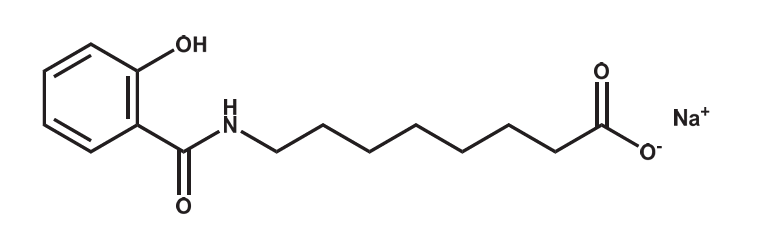
Figure 7: Structural formula
of SNAC
SNAC is an amphiphilic N-acetylated amino acid
derivative of salicylic acid. The oral
administration process can be divided into three steps: "Gastric Erosion," "Drug Release," and
"Drug Absorption," with SNAC playing a vital role in
each:
1. Gastric Erosion: As the tablet erodes in the stomach, SNAC is released in high concentrations to
help neutralize the local pH, reducing pepsin activity and
protecting the peptide.
2. Drug
Release: SNAC
promotes the monomerization of the peptide by weakening hydrophobic
interactions through changes in solution polarity, facilitating
better absorption.
3. Drug Absorption: SNAC integrates into the lipid membrane to increase the fluidity of the gastric epithelium, allowing the
peptide to enter the systemic
circulation
through transcellular pathways.
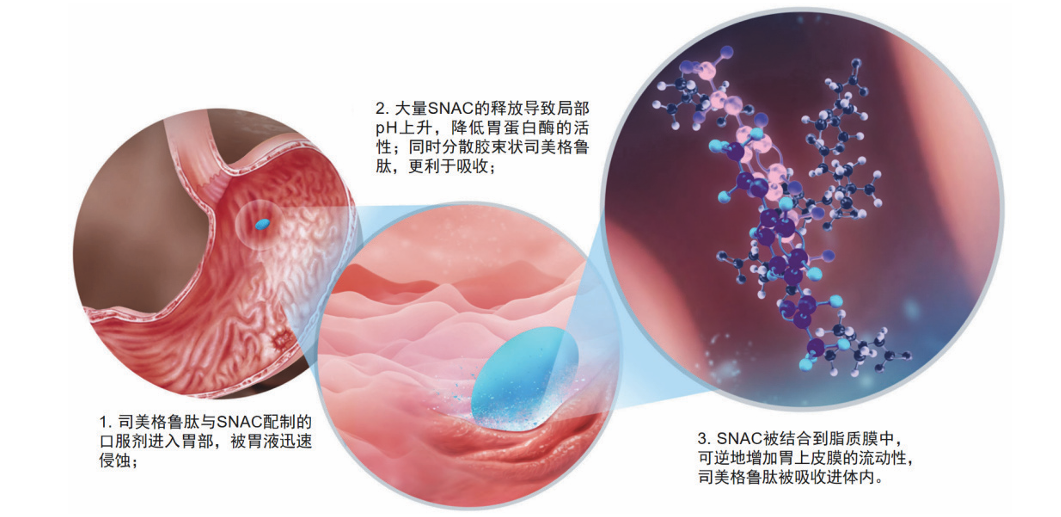
Figure 8: SNAC-assisted oral absorption process of Semaglutide
(Source: Journal of Medicinal
Chemistry)
Translation of Figure 8:
Mechanism of Oral Semaglutide Absorption
1. Gastric Erosion: The oral formulation composed
of Semaglutide and SNAC enters the stomach and is rapidly eroded by gastric juice.
2. Drug Release and Protection: The release of a large amount of SNAC leads to a local rise
in pH, which reduces the activity of pepsin. Simultaneously, it disperses the micellar Semaglutide, making
it more favorable for
absorption.
3. Absorption: SNAC is incorporated into the lipid membrane, reversibly increasing the fluidity of the gastric epithelial membrane. This allows the Semaglutide to be absorbed
into the body and enter the systemic circulation.
Semaglutide's impact on weight loss is particularly significant; clinical practice has shown it delays gastric emptying,
increases satiety, and reduces appetite. The weight-loss-
specific version, Wegovy, was approved by the US FDA in June 2021 for once-weekly 2.4 mg
subcutaneous injections.
However, there are specific criteria for its use:
• BMI > 30 (Obese)
• BMI 27–30 with at least one weight-related comorbidity
Even with proper use,
side efects cannot be ignored; the reported rate of gastrointestinal
adverse events in
clinical trials for Wegovy reached 84.1%, including nausea, diarrhea, and
vomiting. Furthermore, if
misused or used at excessively high doses without medical supervision, there are risks of thyroid C-cell tumors, and in men, risks of erectile
dysfunction
and testosterone deficiency.
"Every medicine has its side
efects." One must not blindly take medication for weight loss, as health is far more valuable than
physical appearance. Compared to
medication, a
healthy diet and diligent exercise remain the most appropriate pathways for weight loss and blood sugar management.
About
CSBio:
For
over 30 years, CSBio, a leading peptide products manufacturing company located
in Silicon Valley, California and with sales offices in China, Europe, India
and Japan, has been providing, R&D & cGMP peptides, peptide reagents,
automated peptide & oligonucleotide instrumentation and related peptides
& oligo services to the global pharmaceutical community. CSBio’s products
and services can be found in production laboratories, universities, and
pharmaceutical companies worldwide.
Interested
in any of our peptide products and services? Please contact us as follows:
Email: info@csbiochina.com
websites: www.csbio.com; www.csbiochina.com; www.csbioshanghai.com
|
 沪公网安备 31011502005552号
沪ICP备13005633号-1
沪公网安备 31011502005552号
沪ICP备13005633号-1